10 Feb 2026
HKMU partners with the University of Sussex to launch Dual Degree Pathway for HKMU Undergraduates


A know-how licensing agreement for a non-invasive electrochemical screening technology, based on research by Prof. A. L. Roy Vellaisamy, Chair Professor of Intelligent Systems in the School of Science and Technology, has been signed with a UK-based healthcare company.

Team members: Prof. Roy Vellaisamy (front), Chair Professor of Intelligent Systems in the School of Science and Technology; Dr Oliver Yeung Chi-shun (back, left), Postdoctoral Fellow; and Mr Cody Mok Yun-kam (back, right), Research Assistant.

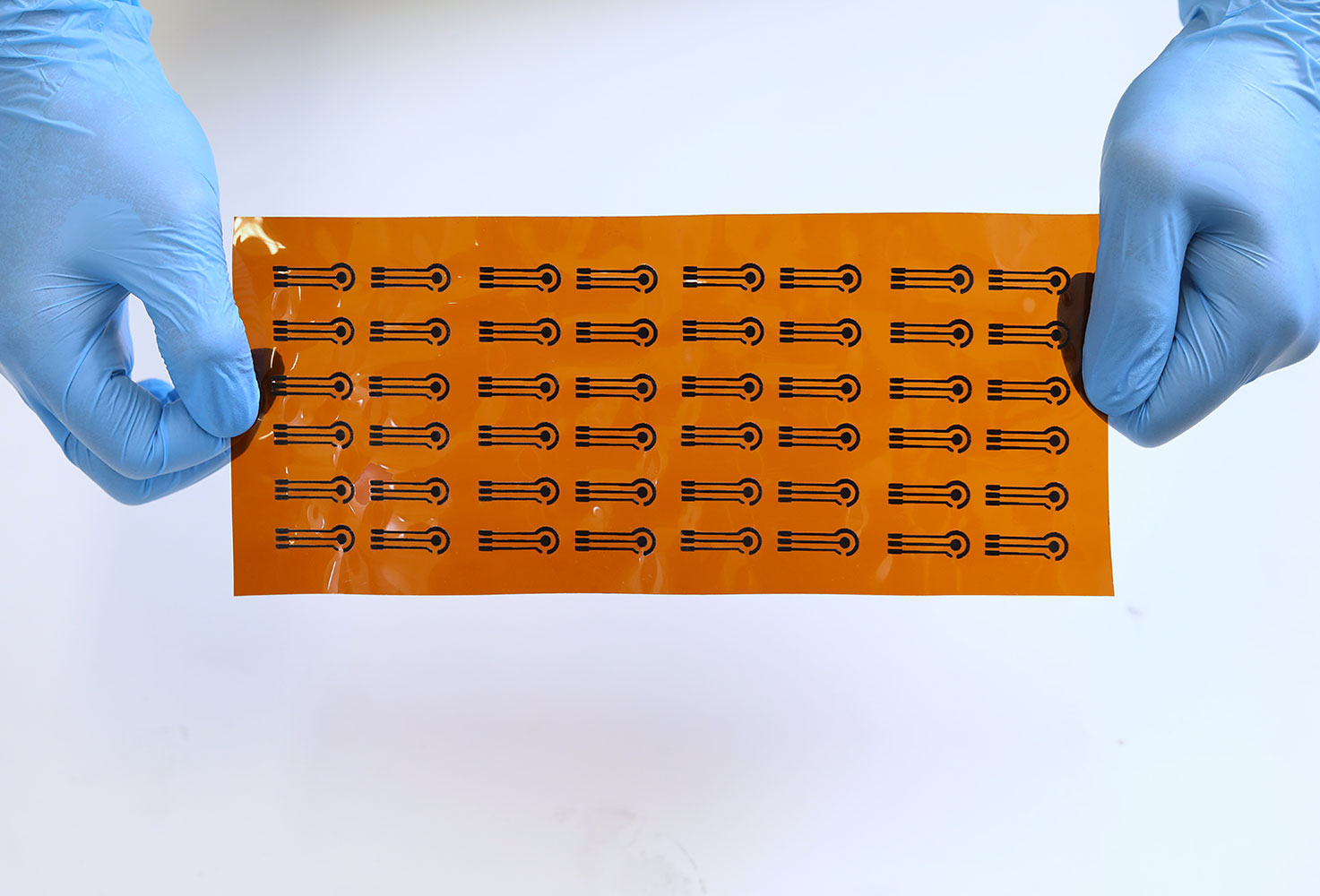
Prof. Vellaisamy and his research team have developed a system integrated with a cost-effective electrode coated with a thin, functionalised layer for the highly selective detection of urinary biomarkers.
Hong Kong Metropolitan University (HKMU) recently achieved its first-ever technology licensing agreement, a milestone in the University's efforts to translate research into impactful, real-world applications. A know-how licensing agreement for a non-invasive electrochemical screening technology, based on foundational research by Prof. A. L. Roy Vellaisamy, Chair Professor of Intelligent Systems in the School of Science and Technology, has been signed with a UK-based healthcare company. The technology offers a highly promising method for the early detection of prostate cancer through a simple urine test.
The licensing agreement with Pinpoint Medical Ltd marks an important step in the company's pathway in the development and commercialisation of a portable, point-of-care diagnostic platform. This platform builds upon technology previously licensed from other universities where Prof. Vellaisamy had worked earlier. Designed to enable clinicians to perform rapid, on-site analysis of urinary biomarkers, the platform aims to facilitate earlier diagnosis and treatment decisions for prostate cancer, ultimately benefiting patients.
A patient-friendly approach to address cancer-screening challenges
Prostate cancer is the second most common cancer and the fifth leading cause of cancer deaths in men worldwide, underscoring the urgent need for more accurate screening techniques for early identification.
While biopsies are considered the “gold standard” for diagnosing prostate cancer, the decision to perform this invasive procedure often depends on the results of initial screening tests. Currently, prostate cancer screening relies on measuring prostate-specific antigen (PSA) levels through a blood test. However, PSA levels can be affected by a range of non-cancer-related factors, such as age, infection and prostate enlargement, making it an unreliable biomarker. Consequently, PSA testing results in high false-positive rates and low specificity, limiting its overall diagnostic accuracy and leading to many unnecessary biopsies, causing patient anxiety and an additional clinical burden.
To address this challenge, Prof. Vellaisamy and his research team developed a novel system for the highly selective detection of urinary biomarkers. At the heart of the system is a cost-effective electrode coated with a thin, functionalised layer. This newly designed layer is composed of a specific mixture of organic monomers and crosslinkers that create recognition sites capable of selectively binding the target molecules. These specially designed and patterned electrodes create a unique three-dimensional transducer, featuring a “complementary morphological structure” that enables the screening of multiple cancer biomarkers simultaneously using advanced machine learning tools.
The reliability of this innovative sensing platform has been successfully demonstrated through performance comparisons with the clinical gold standard for detecting prostate cancer, showing its high potential as a reliable diagnostic tool. Earlier findings from Prof. Vellaisamy's research were published in the scientific journals ACS Omega and ACS Sensors. The new know-how licence from HKMU provides crucial expertise to help advance this promising technology to its next stage of development and commercialisation.
“This technology helps pave the way for developing a screening system that is cost-effective, non-invasive, accurate and user-friendly,” said Prof. Vellaisamy. “By licensing the technology know-how, we hope to apply this innovation to create a point-of-care diagnostic platform for analysing urinary biomarkers, thus minimising the need for painful and invasive procedures, and enhancing the early detection of the prostate cancer.” The technology also has the potential for detecting bladder cancer, he added.
Prof. Ricky Kwok Yu-kwong, Vice President (Research and Institutional Advancement), stated that this licensing agreement marks a significant step in knowledge transfer for HKMU, highlighting the University's commitment to impactful research. “The University has made remarkable progress in research and knowledge transfer in recent years,” he said. “Since filing our first patent in late 2023, we have expanded our portfolio to include several dozen patents. We will continue our efforts to advance applied research and translate our research outcomes into practical solutions that contribute to societal development.”

A know-how licensing agreement for a non-invasive electrochemical screening technology, based on research by Prof. A. L. Roy Vellaisamy, Chair Professor of Intelligent Systems in the School of Science and Technology, has been signed with a UK-based healthcare company.




Hong Kong Metropolitan University (HKMU) recently achieved its first-ever technology licensing agreement, a milestone in the University's efforts to translate research into impactful, real-world applications. A know-how licensing agreement for a non-invasive electrochemical screening technology, based on foundational research by Prof. A. L. Roy Vellaisamy, Chair Professor of Intelligent Systems in the School of Science and Technology, has been signed with a UK-based healthcare company. The technology offers a highly promising method for the early detection of prostate cancer through a simple urine test.
The licensing agreement with Pinpoint Medical Ltd marks an important step in the company's pathway in the development and commercialisation of a portable, point-of-care diagnostic platform. This platform builds upon technology previously licensed from other universities where Prof. Vellaisamy had worked earlier. Designed to enable clinicians to perform rapid, on-site analysis of urinary biomarkers, the platform aims to facilitate earlier diagnosis and treatment decisions for prostate cancer, ultimately benefiting patients.
A patient-friendly approach to address cancer-screening challenges
Prostate cancer is the second most common cancer and the fifth leading cause of cancer deaths in men worldwide, underscoring the urgent need for more accurate screening techniques for early identification.
While biopsies are considered the “gold standard” for diagnosing prostate cancer, the decision to perform this invasive procedure often depends on the results of initial screening tests. Currently, prostate cancer screening relies on measuring prostate-specific antigen (PSA) levels through a blood test. However, PSA levels can be affected by a range of non-cancer-related factors, such as age, infection and prostate enlargement, making it an unreliable biomarker. Consequently, PSA testing results in high false-positive rates and low specificity, limiting its overall diagnostic accuracy and leading to many unnecessary biopsies, causing patient anxiety and an additional clinical burden.
To address this challenge, Prof. Vellaisamy and his research team developed a novel system for the highly selective detection of urinary biomarkers. At the heart of the system is a cost-effective electrode coated with a thin, functionalised layer. This newly designed layer is composed of a specific mixture of organic monomers and crosslinkers that create recognition sites capable of selectively binding the target molecules. These specially designed and patterned electrodes create a unique three-dimensional transducer, featuring a “complementary morphological structure” that enables the screening of multiple cancer biomarkers simultaneously using advanced machine learning tools.
The reliability of this innovative sensing platform has been successfully demonstrated through performance comparisons with the clinical gold standard for detecting prostate cancer, showing its high potential as a reliable diagnostic tool. Earlier findings from Prof. Vellaisamy's research were published in the scientific journals ACS Omega and ACS Sensors. The new know-how licence from HKMU provides crucial expertise to help advance this promising technology to its next stage of development and commercialisation.
“This technology helps pave the way for developing a screening system that is cost-effective, non-invasive, accurate and user-friendly,” said Prof. Vellaisamy. “By licensing the technology know-how, we hope to apply this innovation to create a point-of-care diagnostic platform for analysing urinary biomarkers, thus minimising the need for painful and invasive procedures, and enhancing the early detection of the prostate cancer.” The technology also has the potential for detecting bladder cancer, he added.
Prof. Ricky Kwok Yu-kwong, Vice President (Research and Institutional Advancement), stated that this licensing agreement marks a significant step in knowledge transfer for HKMU, highlighting the University's commitment to impactful research. “The University has made remarkable progress in research and knowledge transfer in recent years,” he said. “Since filing our first patent in late 2023, we have expanded our portfolio to include several dozen patents. We will continue our efforts to advance applied research and translate our research outcomes into practical solutions that contribute to societal development.”




SIGN UP FOR OUR LATEST NEWS
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.